Sunriser® DPI
High Performance, High-Dose Capsule DPI.
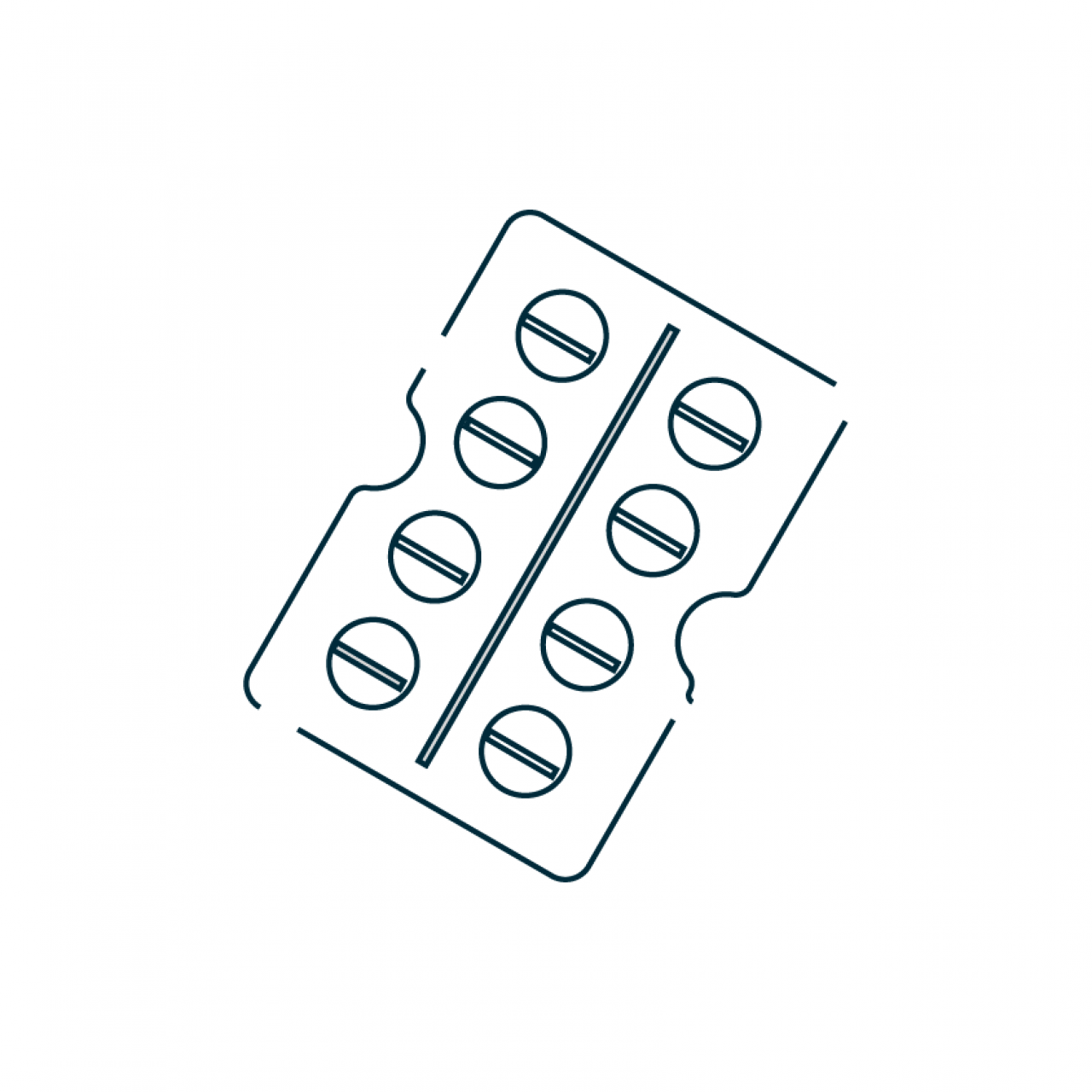
Sunriser® is a patented high-performance capsule-based dry powder inhaler capable of delivering up to 50 mg per dose with >50% lung delivery performance. Developed by Hovione in partnership with H&T Presspart, Sunriser® features a unique capsule movement that enables best-in-class delivery efficiency both with classic carrier-based and with spray-dried engineered formulations.
Sunriser® is developed for indications where chronic or medium-term treatments require high drug loads including the delivery of challenging cohesive and sensitive materials, with maximum efficiency.
Sunriser® offers a portable patient-friendly inhaler solution and is available for Licensing and Supply.
Applications
- Asthma
- COPD
- CF
- IPF
- Antibiotics for lung infection
- Analgesic drugs
- Pulmonary hypertension drugs
Designed for maximum efficiency in Dry Powder Inhalation delivery
The demand for inhalable drugs that require high drug loads and the delivery of cohesive and sensitive materials has increased, making it necessary to develop more efficient delivery solutions. To address these challenges, the Sunriser® incorporates a unique flow path through the device, which combined with high-performance capsule dynamics, drives maximum efficiency in the dispersion and deagglomeration of inhalable dry powder formulations. Sunriser’s patented innovative engine delivers >50% to the lung consistently across flow rates and across formulation types.
Sunriser® is designed to overcome patient usability challenges promoting adherence and compliance with easy capsule loading, audio feedback and a transparent capsule chamber for visual checking that the complete dose has been inhaled from the capsule. It provides classical piercing with two needles and operates with size 3 capsules. Connectivity and digital features can be integrated.
In addition to Sunriser® device supply, Hovione can provide all formulation development services and capsule filling for targeted lung and systemic indications. Hovione can conduct fully integrated device and formulation development and optimization of generic and innovator drug products, from proof-of-concept to clinical manufacturing and commercial drug product supply.
Available for Partnering and Supply
- Multi-use capsule-based dry powder inhaler
- High-performance, fine particle fraction > 50% across flow rates with both carrier-based and spray-dried engineered formulations
- Patent life up to 2038
- Easy-to-use: open and load, close and inhale
- Audio feedback to the patient
- Highly portable and convenient
- Classical capsule piercing with two needles
- Operates with Size 3 capsules
- Transparent capsule chamber for dose feedback to patient
- Dose up to 50 mg
- Medium device resistance
- Color and branding customization available
- Connectivity and digital features can be integrated
- Available for Licensing, Supply and integrated Formulation-Device development
|
|
|
|
|
|
|
Patent life to 2035 |
Blister |
Fully injection moulded |
Economic at Global Scale |
Available for Licensing and Supply |
Contact our experts today to bring your Inhaled medicine to patients
Contact Us
Please contact us if you have inquiries about our offering
https://go.hovione.com/l/47122/2014-08-06/9grc