Quality by Design
Pioneering Quality by Design (QbD) for Process Development.
Hovione, with an extensive experience in process development and a proven track record with more than 100 commercial products in the past 10 years,, has consistently implemented principles of Quality by Design (QbD) for the last two decades. This dedication has enabled us to refine the QbD methodology, leveraging our expertise and experience to streamline the process development stage, and design robust control strategies for new manufacturing processes.
Our QbD approach for process development relies on the integration of four key tools:
- Knowledge Management: the solid history of success in development of new processes has culminated in a comprehensive knowledge management platform that captures and utilizes our accumulated knowledge to support the design of control strategies for new products. This platform encompasses a vast database of process data, first-principle and statistical models, and risk management tools that consolidate the cumulative learnings from our manufacturing experience.
- Process Modeling: We have invested in developing our own mathematical models for over 15 years, enabling us to accurately represent the physical phenomena governing various unit operations in our manufacturing equipment. This predictive capability empowers us to optimize processes, anticipate scale-up risks, and expedite development timelines while reducing costs.
- Laboratory Facilities: Our customized laboratory facilities, equipped with state-of-the-art instrumentation and reliable scale-down models, provide the industrial representative environment required to conduct controlled experiments, gather valuable data, and optimize process parameters with high precision for developing new manufacturing processes.
- Real-Time Process Analytics: We use state-of-the-art process analytical technologies to monitor the processes in real time and maximize the information extracted from each experiment or manufacturing batch. These technologies ensure product quality is maintained throughout production and can be used to enhance the control strategy of new products.
In addition, we are committed to partnering with our clients, leveraging our internal tools, and tailoring our QbD implementation strategy to meet each specific need. Our shared goal is to achieve a predictable and successful scale-up and development process, ensuring the quality standards are maintained throughout the entire life cycle.
“Hovione has a unique set of competences in the area of powder processing. QbD has helped us save over a million dollars in API”
Large Phama, Europe
Quality-by-Design Methodology at Hovione
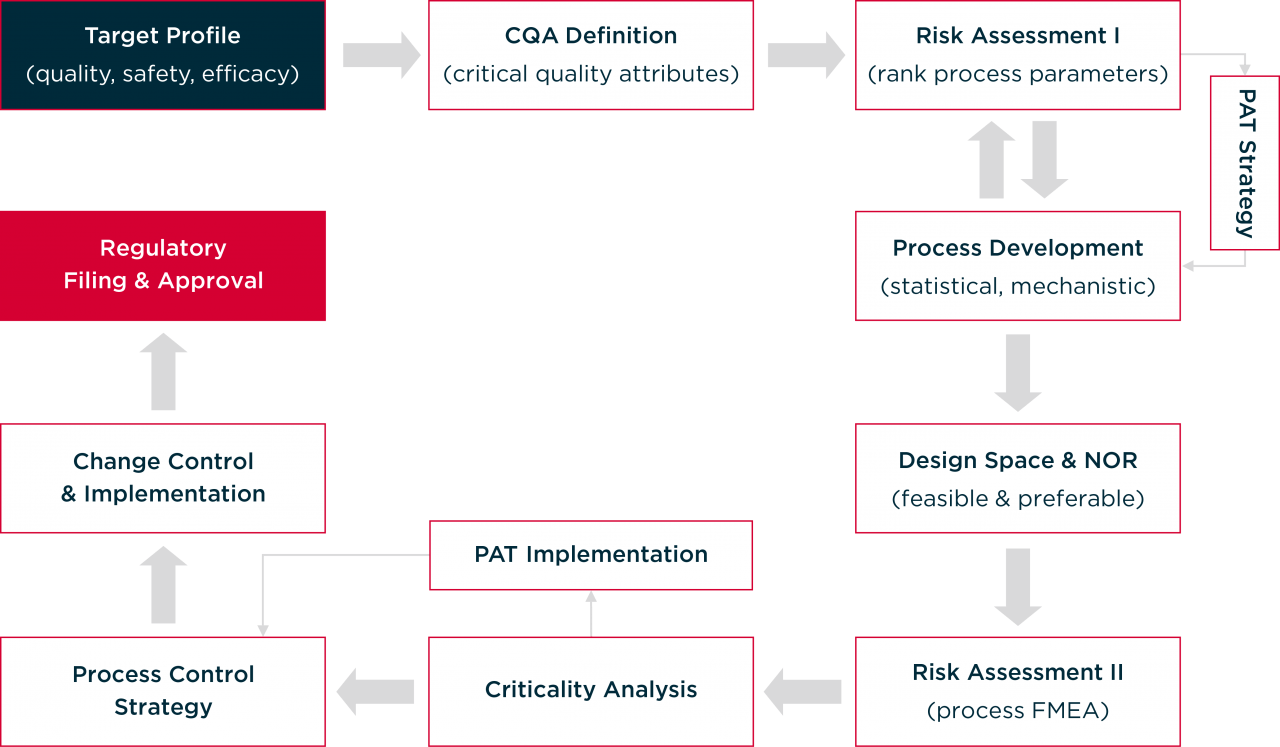
Learn more about QbD, with the following articles by Hovione's experts:
Quality by Design in API process development
Published in Speciality Chemicals Magazine, January 2013
Accelerating approval and reducing costs of spray dried drugs through development by design
Published in Pharma's Almanac, August 2016
