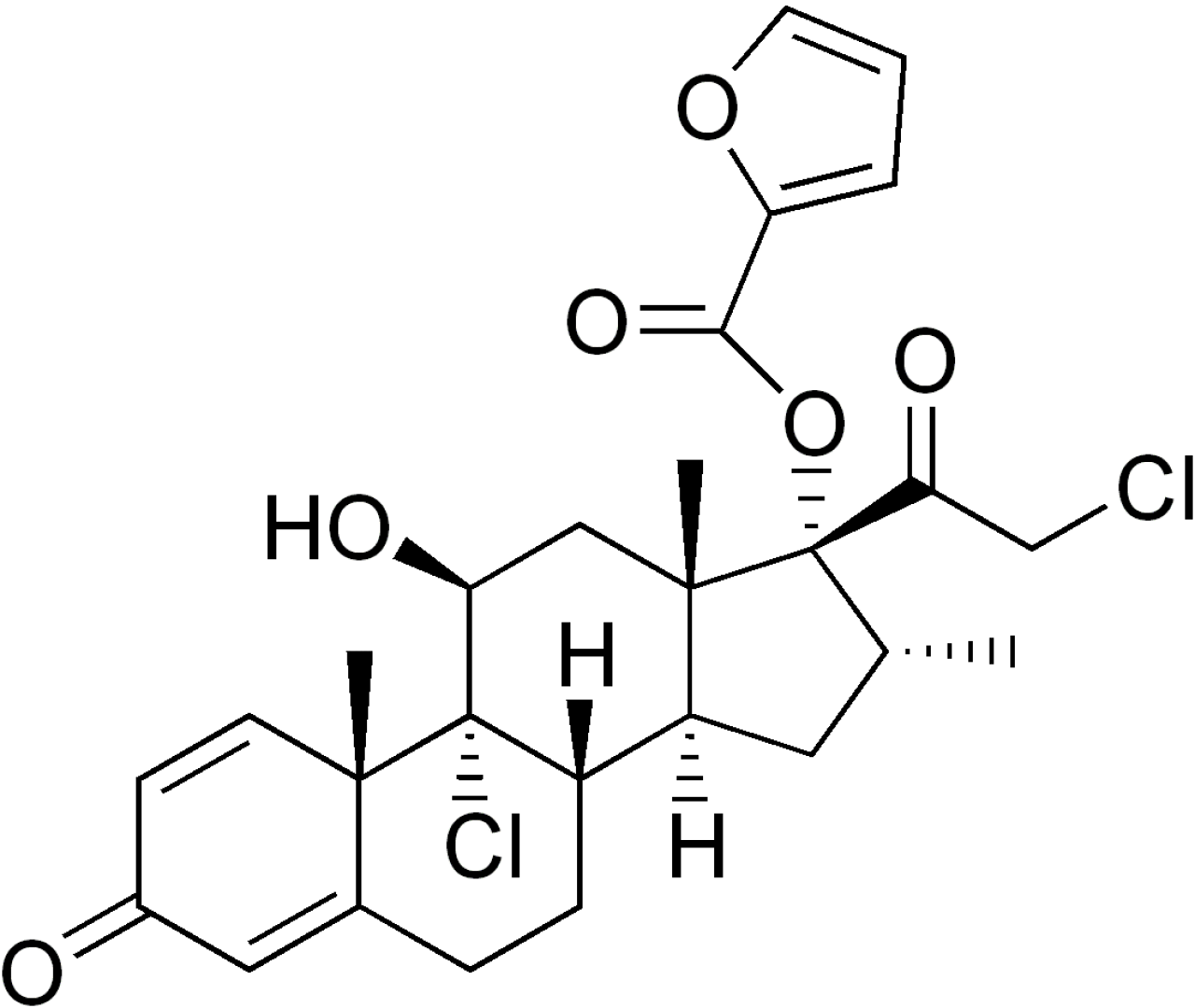
Mometasone Furoate Anhydrous
- Micronized
- Non-Micronized
- Hovione Loures
- Corticosteroids
- Topical
- Inhalation
- Nasal
- Allergic rhinitis
- Asthma
- Skin disorders
Hovione can customize Mometasone Furoate Anhydrous API for inhalation as well as other demanding applications relying on its experience in particle size reduction technologies with tailored made, highly reproducible particle size distributions.
Low amorphous content and high stability with an industry leading impurity profile are also part of our offering.
Hovione Mometasone Furoate Anhydrous API is available since 1999 approved in generic applications.
With a proven track record in developing and manufacturing high performance APIs Hovione offers a full range of API inhalation, e.g. Glycopyrronium Bromide.
This is not to be construed as a representation of non-infringement or as an offer to sell in those countries where such would constitute an infringement of third parties' patent rights.
Contact Us
Please contact us if you have inquiries about our offering
https://go.hovione.com/l/47122/2014-08-06/9grc