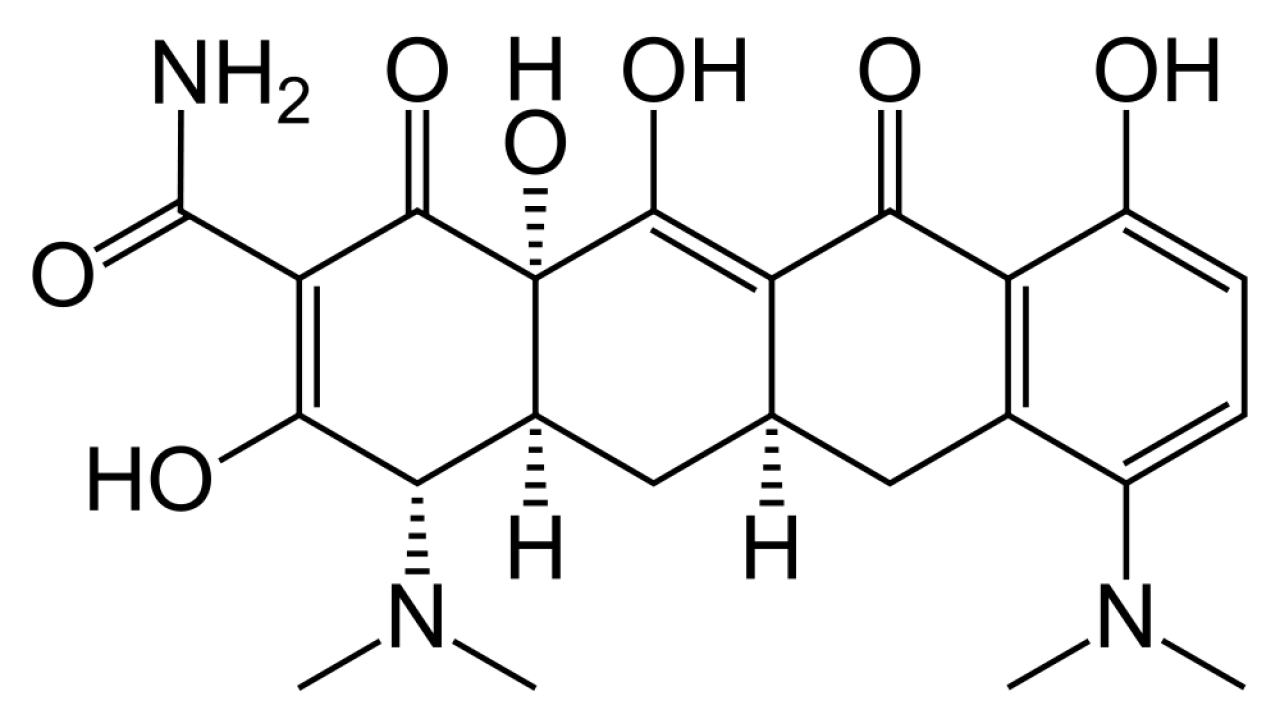
Minocycline Hydrochloride
- Micronized
- Non-Micronized
- Hovione Loures
- Tetracycline Antibiotic
- Topical
- Oral
- Injectable
- Anti-Infectives
- Acute Acne
Hovione is a leading supplier of Minocycline HCl since 1988 with an unblemished regulatory track record.
We have a long lasting strategic commitment to this product that ensures continuity an security of supply.
We can support new Minocycline HCl formulation development with a range of particle sizes that will meet your specific needs.
Hovione Minocycline is approved in generic and 505b2 applications.
This is not to be construed as a representation of non-infringement or as an offer to sell in those countries where such would constitute an infringement of third parties' patent rights.
Contact Us
Please contact us if you have inquiries about our offering
https://go.hovione.com/l/47122/2014-08-06/9grc