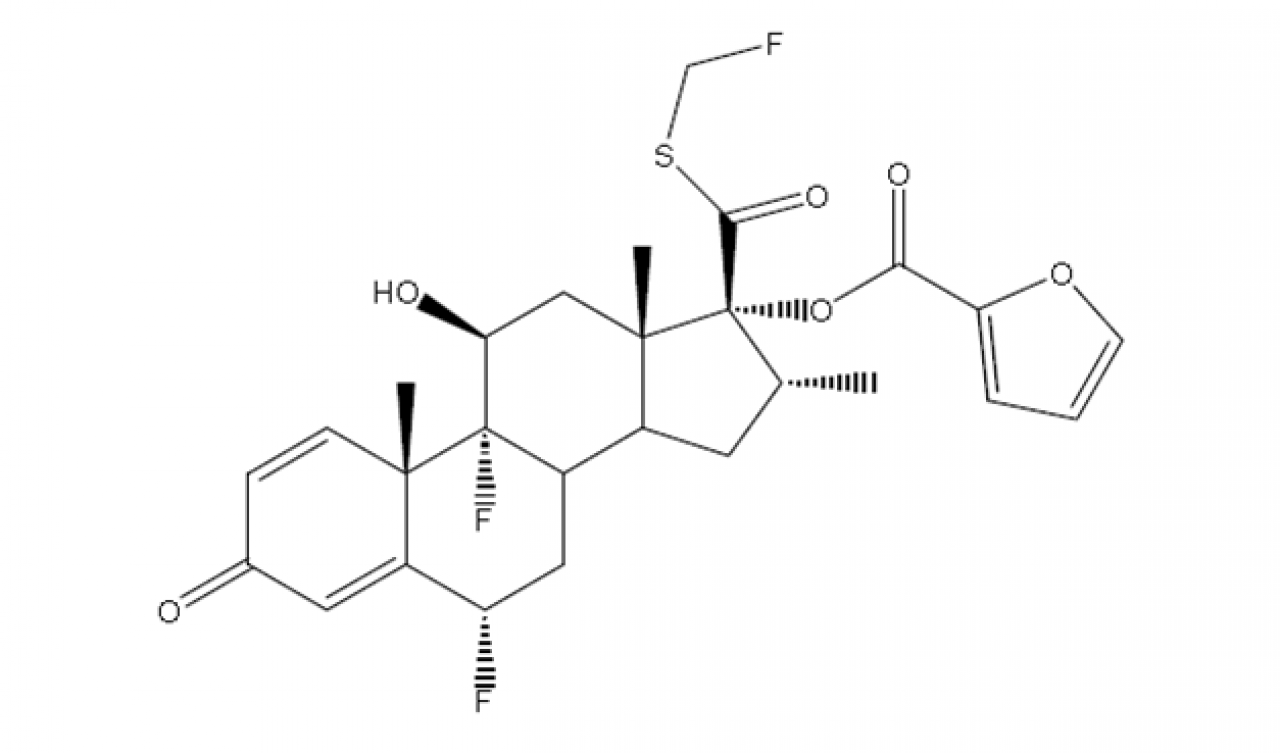
Fluticasone Furoate
- Inhalation
- Hovione Loures
- Corticosteroids
- Inhalation
- Nasal
- COPD
Hovione offer Fluticasone Furoate, crystalline form I for development trials with tailor made particle design to best fit your application, yielding Inhalation grade material, featuring a near-perfect particle size distribution reproducibility and low span batch after batch.
Low amorphous content and high stability with >99.60% purity are also features of this API.
With a proven track record in developing and manufacturing high performance APIs Hovione offers a full range of API inhalation, e.g. Vilanterol Trifenatate or Umeclidinium Bromide.
This is not to be construed as a representation of non-infringement or as an offer to sell in those countries where such would constitute an infringement of third parties' patent rights.
Contact Us
Please contact us if you have inquiries about our offering
https://go.hovione.com/l/47122/2014-08-06/9grc